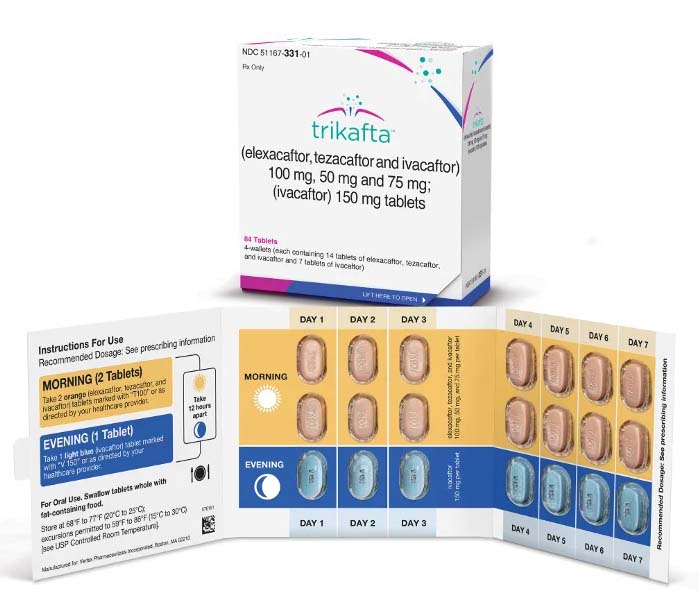
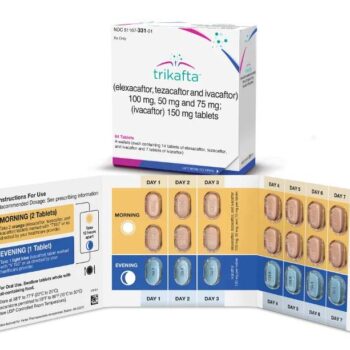
Trikafta is a revolutionary pharmaceutical medication used for the treatment of cystic fibrosis (CF), a genetic disorder that affects the respiratory and digestive systems. It is a combination therapy that includes three active ingredients: elexacaftor, tezacaftor, and ivacaftor. Trikafta is designed to address the underlying genetic mutations in individuals with CF and has been shown to significantly improve lung function and overall health in many patients.
Here is a detailed description of Trikafta:
Active Ingredients:
- Elexacaftor: Elexacaftor is a CFTR modulator medication that targets specific CFTR protein defects. It helps these proteins to function more effectively, improving the transport of salt and fluids in and out of cells. This leads to reduced mucus thickness and improved lung function.
- Tezacaftor: Tezacaftor is another CFTR modulator that complements the action of elexacaftor. It helps the CFTR protein move to the cell surface, where it can function properly. This enhances the regulation of salt and fluid flow and further reduces mucus buildup.
- Ivacaftor: Ivacaftor is a CFTR potentiator medication. It enhances the function of the CFTR protein that has reached the cell surface, increasing chloride ion transport and further improving airway hydration.
Indications:
Trikafta is indicated for the treatment of CF in patients who have specific genetic mutations that are responsive to this combination therapy. It has been shown to be particularly effective in patients with at least one copy of the F508del mutation, among others.
Dosage Forms:
Trikafta is available in the form of oral tablets, which are taken by mouth.
Usage:
The dosing and administration of Trikafta are determined by a healthcare provider and are based on the patient’s age, weight, and specific genetic mutations. It is generally taken as prescribed, typically once or twice daily with food to optimize absorption.
Benefits:
Trikafta offers the potential to transform the lives of individuals with CF by addressing the underlying genetic defects responsible for the disease. It has been shown to significantly improve lung function, reduce respiratory symptoms, and enhance overall quality of life. For many patients, it represents a groundbreaking advancement in CF treatment.
Precautions:
Patients must undergo genetic testing to identify the specific mutations that make them eligible for Trikafta treatment. It is crucial to use Trikafta as directed by a healthcare provider and to be aware of potential side effects and drug interactions.
Side Effects:
Common side effects of Trikafta may include headache, upper respiratory tract infection, abdominal pain, and diarrhea. Patients should discuss any side effects or concerns with their healthcare provider. Serious side effects are possible but less common.
In conclusion, Trikafta (elexacaftor/tezacaftor/ivacaftor; ivacaftor) is a groundbreaking combination medication used to treat cystic fibrosis in individuals with specific responsive mutations. It addresses the underlying genetic defects to improve lung function, reduce respiratory symptoms, and significantly enhance the overall quality of life for many patients with CF. Patients should use it as directed by their healthcare provider and be aware of potential side effects and precautions associated with its use. Trikafta represents a major advancement in the treatment of cystic fibrosis and offers hope for improved outcomes for those affected by this condition.





Reviews
There are no reviews yet.