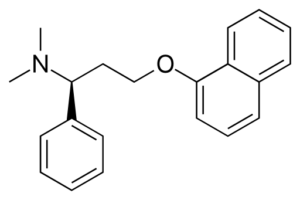
Chemical name: (1S)-N,N-dimethyl-3-naphthalen-1-yloxy-1-phenylpropan-1-amine
Formula: C21H23NO
Anabolic activity index: not a steroid
Androgenic activity index: not a steroid
POXET-30
Overview:
POXET-30 is a medication used primarily for the treatment of premature ejaculation (PE) in adult men. It contains dapoxetine hydrochloride, a selective serotonin reuptake inhibitor (SSRI) that is specifically designed to treat PE. POXET-30 helps to increase the time it takes to ejaculate and improve control over ejaculation.
Mechanism of Action:
Dapoxetine, the active ingredient in POXET-30, works by inhibiting the serotonin transporter, increasing serotonin’s action at the postsynaptic cleft, and delaying ejaculation reflex.
Indications:
POXET-30 is indicated for the treatment of premature ejaculation (PE) in adult men aged 18 to 64 years. It is suitable for men who experience persistent or recurrent ejaculation with minimal sexual stimulation before, on, or shortly after penetration, and before the patient wishes, and that it causes marked distress or interpersonal difficulty.
Administration and Dosage:
The recommended starting dose for most patients is 30 mg, taken as needed approximately 1 to 3 hours prior to sexual activity. POXET-30 should be swallowed whole with a full glass of water. It can be taken with or without food. Patients should not exceed one tablet within a 24-hour period.
Contraindications:
POXET-30 is contraindicated in patients with a hypersensitivity to dapoxetine hydrochloride or any of the excipients. It should not be used in patients with significant pathological cardiac conditions, such as heart failure, conduction abnormalities, significant ischemic heart disease, or valvular disease.
Warnings and Precautions:
Patients should be cautioned against using POXET-30 concomitantly with other SSRIs, monoamine oxidase inhibitors (MAOIs), thioridazine, or within 14 days of discontinuing treatment with an MAOI. POXET-30 should not be used in patients with a history of mania/hypomania or bipolar disorder.
Adverse Reactions:
Common adverse reactions associated with the use of POXET-30 include nausea, dizziness, headache, insomnia, and fatigue. Other less common adverse effects may include dry mouth, diarrhea, abdominal pain, and blurred vision. Patients should be advised to seek medical attention if they experience persistent or troublesome adverse effects.
Storage:
Store POXET-30 tablets at controlled room temperature (25°C/77°F); excursions permitted to 15-30°C (59-86°F). Protect from moisture and light. Keep out of reach of children.
Conclusion:
POXET-30 is a reliable treatment option for men suffering from premature ejaculation, offering an effective way to improve control over ejaculation and enhance sexual satisfaction. It is important for patients to follow dosage instructions and heed precautions to ensure safe and effective use.





Reviews
There are no reviews yet.