Overview
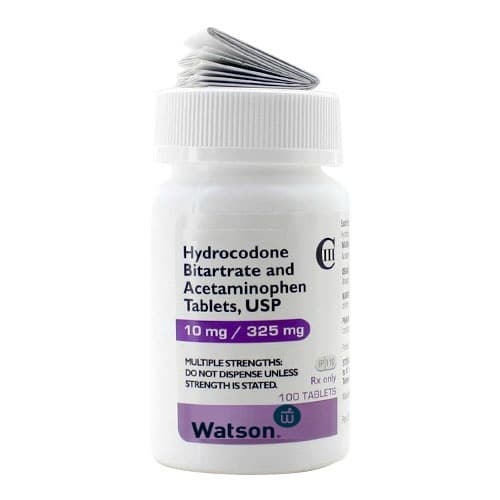
Hydrocodone Bitartrate and Acetaminophen Tablets USP are a combination of two active ingredients: hydrocodone bitartrate, an opioid pain medication, and acetaminophen, a non-opioid pain reliever. This medication is designed to provide relief from moderate to severe pain.
Composition
- Hydrocodone Bitartrate: 10 mg
- Acetaminophen: 325 mg
Each tablet contains 10 mg of hydrocodone bitartrate and 325 mg of acetaminophen.
Mechanism of Action
- Hydrocodone Bitartrate: This opioid analgesic works by binding to the mu-opioid receptors in the brain and spinal cord, altering the perception and response to pain.
- Acetaminophen: This analgesic and antipyretic works by inhibiting the synthesis of prostaglandins in the brain and blocking pain impulse generation.
Indications
Hydrocodone Bitartrate and Acetaminophen Tablets are indicated for the relief of moderate to moderately severe pain. This combination medication is generally prescribed when other pain treatments have not been effective or are not tolerated.
Dosage and Administration
- Adults: The usual recommended dose is one tablet every 4 to 6 hours as needed for pain. The total daily dosage should not exceed 6 tablets.
- Pediatric Patients: Not recommended for children under the age of 18.
Warnings and Precautions
- Opioid Addiction, Abuse, and Misuse: Hydrocodone bitartrate is a Schedule II controlled substance with a high potential for abuse and addiction.
- Respiratory Depression: Can occur with the use of opioids, especially in elderly patients, those with pre-existing respiratory conditions, or when used in combination with other central nervous system depressants.
- Hepatotoxicity: Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death, usually at doses that exceed 4,000 mg per day.
- CNS Depression: Can impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
- Pregnancy and Lactation: Use during pregnancy can result in neonatal opioid withdrawal syndrome. Acetaminophen crosses the placenta and is excreted in breast milk.
Contraindications
- Patients with significant respiratory depression
- Acute or severe bronchial asthma or hypercarbia
- Known hypersensitivity to hydrocodone or acetaminophen
Side Effects
- Common: Nausea, vomiting, dizziness, lightheadedness, sedation, constipation
- Serious: Respiratory depression, severe hypotension, adrenal insufficiency, serotonin syndrome, severe skin reactions
Drug Interactions
- CYP3A4 Inhibitors: Can increase hydrocodone plasma concentrations.
- CYP3A4 Inducers: Can decrease hydrocodone plasma concentrations.
- CNS Depressants: Increased risk of respiratory depression, sedation, and hypotension.
- Serotonergic Drugs: Risk of serotonin syndrome.
Storage
Store at 20° to 25°C (68° to 77°F) in a tight, light-resistant container.
Packaging
Each bottle contains 100 tablets of Hydrocodone Bitartrate and Acetaminophen, 10 mg / 325 mg.
Important Note
This product should be used strictly as prescribed by a healthcare professional. Misuse of opioid medications can lead to addiction, overdose, or death.
For any questions or concerns about this medication, please consult your healthcare provider.






Reviews
There are no reviews yet.