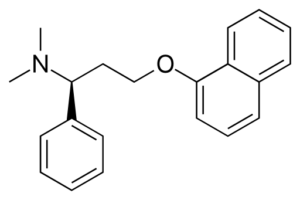
Chemical name: (1S)-N,N-dimethyl-3-naphthalen-1-yloxy-1-phenylpropan-1-amine
Formula: C21H23NO
Anabolic activity index: not a steroid
Androgenic activity index: not a steroid
DURATIA-30
Introduction: DURATIA-30, manufactured by Fortune Health Care, is a medication specifically formulated to treat premature ejaculation (PE) in adult men. Each tablet contains 30 mg of Dapoxetine hydrochloride, the active pharmaceutical ingredient known for its efficacy in prolonging the time to ejaculation and improving control over ejaculation. This product is available in blister packs containing 10 tablets of 30 mg each.
Active Ingredient: The active ingredient in DURATIA-30 is Dapoxetine hydrochloride, a selective serotonin reuptake inhibitor (SSRI) that is approved for the treatment of premature ejaculation. Unlike other SSRIs used for depression, Dapoxetine has a rapid onset of action and is quickly absorbed after oral administration. It works by increasing the activity of serotonin in the nervous system, which helps to delay the reflex that triggers ejaculation.
Mechanism of Action: Dapoxetine acts on the serotonin transporter, inhibiting the reuptake of serotonin. This leads to increased serotonin levels in the synaptic cleft, which in turn enhances the neurotransmission and delays ejaculation. By modulating the neurotransmission in the brain, Dapoxetine helps to improve control over ejaculation and extend the duration of sexual intercourse.
Dosage and Administration: DURATIA-30 tablets are intended for oral administration and should be swallowed whole with a glass of water. The recommended starting dose is 30 mg, taken approximately 1 to 3 hours before anticipated sexual activity. It is important to take the tablet only when needed and not exceed one tablet per day. The efficacy and safety of DURATIA-30 have been demonstrated in clinical trials involving men with premature ejaculation.
Pharmacokinetics: Dapoxetine hydrochloride is rapidly absorbed after oral administration, with peak plasma concentrations occurring within 1 to 2 hours. The elimination half-life of Dapoxetine is approximately 1 to 1.5 hours, making it suitable for on-demand use. The metabolism of Dapoxetine primarily occurs in the liver via the CYP2D6 and CYP3A4 enzymes, and the metabolites are eliminated primarily in the urine. This pharmacokinetic profile allows for flexibility in timing sexual activity without the need for daily dosing.
Efficacy in Premature Ejaculation: Clinical studies have consistently shown that Dapoxetine significantly improves the control over ejaculation and extends the time to ejaculation in men with premature ejaculation. Patients treated with Dapoxetine reported increased satisfaction with sexual intercourse and improved overall sexual function. The efficacy of DURATIA-30 has been demonstrated across different populations, including those with underlying medical conditions such as diabetes and hypertension.
Safety Profile: DURATIA-30 is generally well-tolerated, with the most common adverse effects being nausea, headache, dizziness, and diarrhea. These side effects are usually mild to moderate in severity and transient. Dapoxetine is contraindicated in patients with a history of hypersensitivity to Dapoxetine or any of the tablet’s components. It should not be used in combination with other SSRIs or medications for erectile dysfunction without medical supervision.
Drug Interactions: Dapoxetine has the potential to interact with other medications, particularly those that affect the cytochrome P450 enzymes involved in its metabolism. Concomitant use of Dapoxetine with potent CYP3A4 inhibitors (e.g., ketoconazole, ritonavir) or CYP2D6 inhibitors (e.g., fluoxetine, paroxetine) should be avoided due to the risk of increased plasma concentrations and potential adverse effects. Patients should inform their healthcare provider about all medications they are taking, including over-the-counter and herbal supplements.
Contraindications: DURATIA-30 is contraindicated in patients with significant cardiovascular disorders, including heart failure, arrhythmias, and recent history of myocardial infarction. It should not be used in patients with moderate to severe hepatic impairment or renal impairment. Dapoxetine is not indicated for use in women or individuals under 18 years of age.
Storage: Store DURATIA-30 tablets at room temperature (25°C) in a dry place away from direct sunlight and moisture. Keep the tablets in their original packaging to protect them from degradation. Keep out of reach of children and pets.
Conclusion: DURATIA-30 (Dapoxetine 30mg Tablets) manufactured by Fortune Health Care offers an effective treatment option for men suffering from premature ejaculation. With its rapid onset of action, favorable pharmacokinetic profile, and demonstrated efficacy in clinical trials, DURATIA-30 provides men with improved control over ejaculation and enhanced sexual satisfaction. It is important for patients to follow the prescribed dosage and consult a healthcare provider for personalized advice on its use.




Reviews
There are no reviews yet.