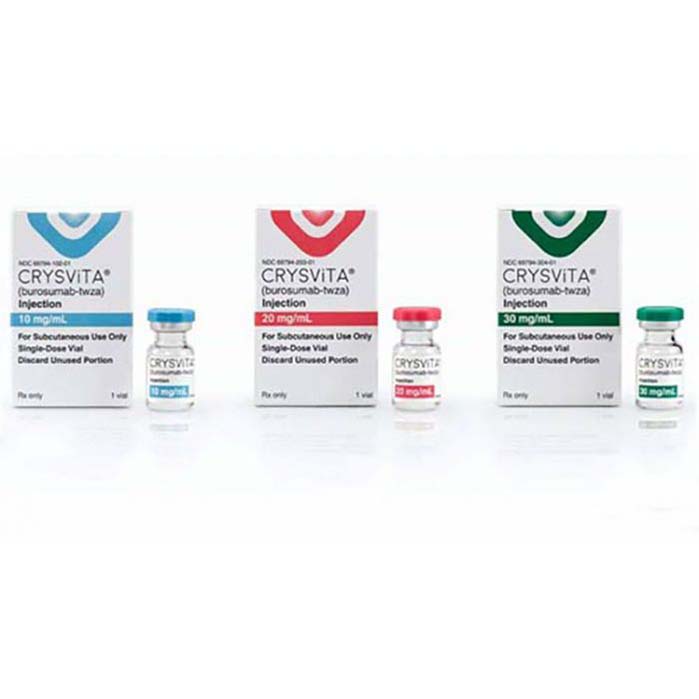
Crysvita (burosumab) is a prescription medicine used to treat adults and children 6 months of age and older with X-linked hypophosphatemia (XLH). XLH is a rare genetic disorder that causes the body to lose too much phosphorus. Phosphorus is a mineral that is essential for strong bones and muscles. People with XLH have low levels of phosphorus in their blood and bones, which can lead to a number of health problems, including:
- Rickets (softening and weakening of the bones) in children
- Osteomalacia (softening and weakening of the bones) in adults
- Muscle weakness
- Pain in the bones and joints
- Fatigue
Crysvita works by blocking the action of fibroblast growth factor 23 (FGF23), a hormone that causes the body to lose phosphorus. By blocking FGF23, Crysvita helps to increase and maintain phosphorus levels in the blood and bones. This can help to improve bone health, reduce pain and muscle weakness, and improve quality of life.
Crysvita is given as an injection under the skin (subcutaneous injection) once every two weeks. The dose of Crysvita is based on the patient’s weight and age.
Crysvita is generally safe and well-tolerated. The most common side effects are mild and go away on their own. These side effects can include:
- Injection site reactions
- Headache
- Fatigue
- Nausea
- Constipation
In rare cases, Crysvita can cause more serious side effects, such as:
- Allergic reactions
- High levels of calcium in the blood
- Jaw problems
Crysvita is a major breakthrough in the treatment of XLH. It is the first and only FDA-approved treatment that specifically targets the underlying cause of XLH. Crysvita has been shown to be effective in improving bone health, reducing pain and muscle weakness, and improving quality of life in people with XLH.
If you or your child has XLH, talk to your doctor about whether Crysvita is right for you.




Joseph Allen –
We saw significant improvements within just a few months of starting Crysvita for our son. His leg bowing has lessened, he’s more active, and his phosphate levels have normalized for the first time. It’s easy to administer through our clinic, and the results speak for themselves.
Melissa Greene –
Crysvita has completely transformed our child’s experience living with X-linked hypophosphatemia. Before starting treatment, she struggled with bone pain and difficulty walking. Since beginning Crysvita, her mobility, energy, and growth have all improved. It’s truly amazing to see her thrive—worth every step in the process.