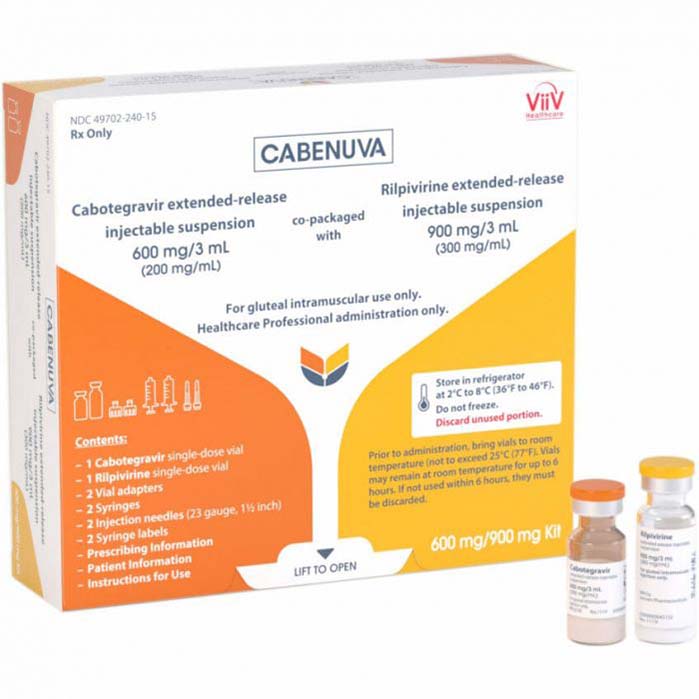
Cabenuva is a combination medication used for the treatment of HIV-1 infection in adults who have already achieved viral suppression with a stable antiretroviral regimen. It is a novel therapy that offers a long-acting, injectable option for maintaining HIV suppression, offering potential benefits in terms of convenience and adherence. Here is a detailed description of Cabenuva (cabotegravir + rilpivirine):
1. Mechanism of Action: Cabenuva is a combination of two active ingredients: cabotegravir and rilpivirine. These medications work together to inhibit the replication of the human immunodeficiency virus (HIV-1).
- Cabotegravir is an integrase strand transfer inhibitor (INSTI). It blocks the activity of the HIV-1 integrase enzyme, preventing the viral DNA from integrating into the host cell’s DNA. This step is crucial for HIV replication.
- Rilpivirine is a non-nucleoside reverse transcriptase inhibitor (NNRTI). It inhibits the reverse transcriptase enzyme, which is responsible for converting viral RNA into DNA, a necessary step in the HIV life cycle.
2. Indications: Cabenuva is indicated as a complete regimen for the treatment of HIV-1 infection in adults to replace their current antiretroviral regimen if their virus has been suppressed (viral load less than 50 copies per mL) on a stable antiretroviral regimen for at least six months with no history of treatment failure.
3. Administration: Cabenuva is administered by intramuscular injection once a month. It is given as two separate injections: one containing cabotegravir and the other containing rilpivirine. The injections are typically administered in the buttocks by a healthcare professional.
4. Effectiveness: Clinical studies have shown that Cabenuva is effective in maintaining viral suppression in people living with HIV-1. It provides an alternative to daily oral regimens, potentially improving adherence and treatment outcomes.
5. Safety Profile: As with any medication, Cabenuva may have potential side effects. Common side effects can include injection site reactions, headache, and fatigue. Serious side effects are rare but can include severe allergic reactions. Patients should discuss potential side effects and any concerns with their healthcare providers.
6. Monitoring: Patients receiving Cabenuva will require regular monitoring of their HIV viral load, CD4 cell count, and overall health to ensure that their HIV infection remains well-controlled.
7. Contraindications: Cabenuva is generally not recommended for individuals with known hypersensitivity to the active ingredients or any of the components in the medication. It is also not recommended for those with HIV-1 strains that are resistant to cabotegravir or rilpivirine.
8. Convenience: One of the primary benefits of Cabenuva is its long-acting injectable format, which reduces the need for daily pill-taking. This can be especially convenient for individuals who prefer or struggle with oral medications.
Cabenuva represents a significant advancement in HIV treatment by offering a novel and effective option for maintaining viral suppression with less frequent dosing. It is crucial for individuals living with HIV-1 to consult with a healthcare provider experienced in HIV care to determine the most appropriate treatment regimen for their specific condition and needs.





Reviews
There are no reviews yet.